Sage
Proven results...solving real problems in healthcare
We create innovative solutions to help improve patient outcomes and caregiver efficiency across the continuum of care. Our products are designed to address preventable never-events, while maximizing efficiency and profitability for healthcare facilities. Our products bring caregivers confidence by helping reduce the risk of hospital-acquired conditions such as skin injury due to incontinence and pressure injuries, pneumonia, and healthcare worker injury. We are driven to solve real problems and make healthcare better for our customers and the patients they serve.


We make products that clinicians use on critically ill patients. That means quality is non-negotiable. It’s what drives us and guides us to always do what’s right.
Our focus on the power of prevention helps you advance patient care by addressing risk factors that can lead to hospital-acquired infections, skin and patient handling injuries.
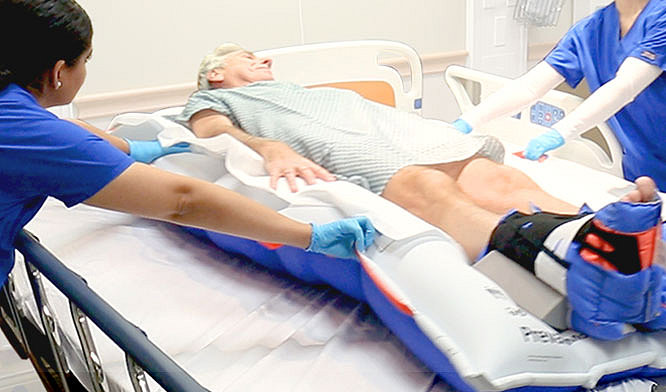
We believe in providing you with the support you need to deliver the best possible care to your patients.
- Implementation planning
- Benchmarking and compliance reporting
- Education and training
- CustomerOne partnership
- FocusRN accredited learning portal
28699
